Abstract
Introduction: Bosutinib is a Src/Abl tyrosine kinase inhibitor (TKI) currently licensed for patients with Ph+ chronic-phase (CP), accelerated-phase (AP), or blast-phase (BP) Chronic Myeloid Leukemia after failure or intolerance to at least 2 other TKIs. It can also be prescribed, in accordance with label if, after failure of the first TKI therapy, another option does not seem feasible. Previous studies demonstrated a potent activity of second-line bosutinib across a spectrum of BCR-ABL1 mutations and a distinct toxicity profile compared to other TKIs.
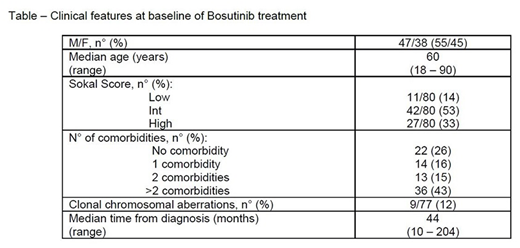
Aim: We retrospectively assessed the efficacy and safety of real life Bosutinib administration in 85 patients resistant/refractory or intolerant to other TKIs, referred to 22 Italian Hematological Institutions. The main features at Bosutinib start are reported in the Table.
Results: At Bosutinib start, all patients were in CP. 60 patients (71%) were resistant/refractory, 25 (29%) were intolerant to previous TKIs. BCR-ABL1 kinase mutations were reported in 5/35 evaluable patients (14%); no T315I was reported. The number of previous TKIs was less than 3 in 24/85 patients (28%) and 3 or more in 61 (72%). Comorbidities were present in 63 patients (74%), the most common being arterial hypertension (40), diabetes (14), cardiac disease (10) and chronic obstructive pulmonary disease (6). The most frequent concomitant medications were: antihypertensive in 55% patients, antiplatelets in 26%, antidiabetic in 19%.
The initial dose was 100 mg in 4/85 patients (5%), 200 mg in 24 (28%), 300 mg in 18 (22%), 400 in 8 (9%); 31 patients (36%) started at the full standard dose of 500 mg.
Major Molecular Response (MMR)/Deep Molecular Response (DMR) were achieved by 37/80 (46%) evaluable patients; 43/80 patients (54%) never reached a MMR/DMR at any time. Best responses in these cases were: Complete Hematologic Response (CHR) in 4 patients (9%), Partial Cytogenetic Response (PCyR) in 6 (14%), Complete Cytogenetic Response (CCyR) in 10 (23%). Dose reductions were necessary in 4 patients (5%) due to adverse events (2 cases of diarrhea, 1 transaminase elevation, 1 skin rash).
After a median follow-up of 26 months (range 3-49), 75/85 patients (88%) are alive and 54/75 patients (72%) are still in treatment. Discontinuations were due to: intolerance in 8/75 patients (11%), loss of response in 3/75 (4%), resistance to therapy in 10/75 (13%). Hematological toxicity was observed in 14 patients (16%), any grade, and grade 3-4 in 3 (3%). Extra-hematological toxicity was reported in 41/85 patients (48%), any grade, and grade 3-4 in 14 patients (16%). Causes of death were: acute myocardial infarction (3), cardiac failure (2), acute respiratory distress (2), allogeneic transplant complications (1), cerebral hemorrhage (2).
Conclusions: In our "real life experience" with Bosutinib, we confirm a stable long-term efficacy in heavily pre-treated, mainly elderly patients with intolerance/resistance to other TKIs, who show an initial response to treatment, as reported in clinical trials. Patients show a fast response to the drug (mainly at three months). Hematological and extrahematological toxicities are manageable.
Breccia:BMS: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Novartis: Honoraria. Abruzzese:Novartis: Consultancy; Ariad: Consultancy; BMS: Consultancy; Pfizer: Consultancy. Castagnetti:Bristol Meyers Squibb: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Bonifacio:Novartis: Research Funding; Amgen: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Bristol Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.